HBME-1 (HBME-1) Mouse Monoclonal Antibody
Specialties: Cytopathology Head/Neck Pathology
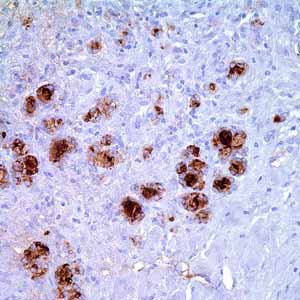
Hector Battifora mesothelial-1 (HBME-1) is a membrane antigen that exists in the microvilli of mesothelial cells and other epithelial cells.1 Anti-HBME-1 labels thyroid papillary carcinoma and follicular carcinoma but not normal thyroid making it a valuable marker for distinguishing thyroid malignacies from benign thyroid lesions.2 It has also been demonstrated to label mesothelial cells, both benign and malignant (malignant mesothelioma) and thus can aid in the identification of mesothelioma.2
- Cheung CC, et al. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001; 14:338-42
- Bateman AC, et al. Immunohistochemical phenotype of malignant mesothelioma: predictive value of CA125 and HBME-1 expression. Histopathology. 1997; 30:49-56.
Specifications
- Reactivity: paraffin
- Control: Mesothelioma (Cytoplasmic, Membranous)
- Dilution Range: 1:25-1:100 *
Package Inserts
IFU
- IVD Rev. 7.0 (CMC28321070)
Have a different keycode?
Click Here
Learn how to obtain your SDS
Ordering Information
For in vitro diagnostic (IVD) use in USA
| 0.1 mL concentrate | 283M-14 |
| 0.5 mL concentrate | 283M-15 |
| 1.0 mL concentrate | 283M-16 |
| 1.0 mL predilute ready-to-use | 283M-17 |
| 7.0 mL predilute ready-to-use | 283M-18 |
For in vitro diagnostic (IVD) use in Canada
| 0.1 mL concentrate | 283M-14 |
| 0.5 mL concentrate | 283M-15 |
| 1.0 mL concentrate | 283M-16 |
| 1.0 mL predilute ready-to-use | 283M-17 |
| 7.0 mL predilute ready-to-use | 283M-18 |
For in vitro diagnostic (IVD) use in Europe
| 0.1 mL concentrate | 283M-14 |
| 0.5 mL concentrate | 283M-15 |
| 1.0 mL concentrate | 283M-16 |
| 1.0 mL predilute ready-to-use | 283M-17 |
| 7.0 mL predilute ready-to-use | 283M-18 |
For research use only (RUO) in Japan
| 0.1 mL concentrate (RUO) | 283M-14-RUO |
| 0.5 mL concentrate (RUO) | 283M-15-RUO |
| 1.0 mL concentrate (RUO) | 283M-16-RUO |
| 1.0 mL predilute ready-to-use (RUO) | 283M-17-RUO |
| 7.0 mL predilute ready-to-use (RUO) | 283M-18-RUO |
To request information on this product in additional countries, please click the button below.