Project Life Cycles for IHC Antibody Development Services
At Cell Marque™ Tissue Diagnostics, our primary focus has always been on helping our customers succeed in their medical endeavors. We understand that there are times when expert assistance is essential to achieving your goals, and that’s where our partnership can ensure your success. With over 30 years of experience, we have supported numerous clients including laboratories and manufacturing sites, in meeting their antibody development needs by actively listening, understanding their challenges, and providing tailored services that drive success.
Below, you will find three of the most popular project life cycles where our experts have effectively managed custom antibody projects, each designed to address specific needs:
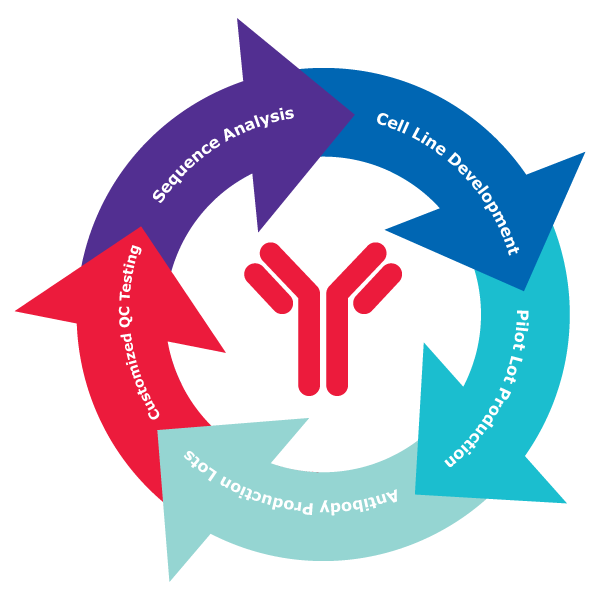
1. Sequence is Given
Overview: When starting with a specific sequence, we guide you through a comprehensive development process tailored to your needs.
Project Life Cycle Steps:
- Sequence Analysis: We begin with a detailed analysis of the provided sequence to establish optimal antibody design.
- Cell Line Development: Our experts create a stable cell line that expresses the target antigen, ensuring high-quality antibody production.
- Pilot Lot Production: A pilot lot is produced to assess the antibody's performance and yield before scale-up.
- Antibody Production Lots: Following successful pilot testing, we proceed to large-scale production of unique lots to demonstrate consistency across lots.
- Customized QC Testing: Each batch undergoes rigorous quality control testing tailored to the customer’s specifications.
2. Established Customer Hybridoma
Overview: When the customer provides an existing hybridoma, we provide a streamlined process to maximize the yield and performance of the resulting antibody.
Project Life Cycle Steps:
- Hybridoma Characterization: We start with a thorough characterization of the hybridoma to confirm its specificity and stability.
- Cell Bank Creation: A master cell bank is created to ensure the availability of hybridoma for future production needs.
- Pilot Lot Production: We produce a pilot lot to evaluate the antibody's performance.
- Production Lots: Subsequent to successful validation, we scale up to produce larger batches, maintaining high-quality standards.
- Customized QC Testing: Tailored quality control testing is performed to ensure the antibodies meet the specific requirements for intended applications.
3. Existing Antibody
Overview: When utilizing an existing antibody, we offer specialized services to validate and optimize your antibodies for adjacent applications, particularly in IHC.
Project Life Cycle Steps:
- Custom QC Testing: We conduct a series of quality control tests to assess the existing antibody's sensitivity and specificity performance in IHC applications.
- Validation for Adjacent Applications: Our team evaluates the antibody's compatibility with various IHC protocols, to identify the best possible match for the desired application.
- Recommendations for Optimization: Based on the testing results, we provide a final report of our findings and any recommendations for optimizing the antibody, which may include adjustments in dilution, incubation times, or buffer systems.
Why partner with us?
Partnering with us for IHC Antibody Development Services means engaging a wealth of knowledge and expertise at every step of the project life cycle. Our commitment to quality and customer satisfaction assures us that your projects are in capable hands, ready to meet the demands of your application needs.
Our Service Offerings Include:
- Hybridoma cell line production
- Stable CHO cell line production
- Scalability from bioreactor to fermentation at multiple volumes
- GMP Cell Banking
- Custom growth media options
- Serum Free Adaptation
- Recombinant Antibody Production
- Fermentation & Purification Optimization
- Custom QC options
- Transient Transfection Expression
- Novel B-Cell Cloning Platform
- Antibody Yield & Performance Optimization
- Reverse Engineering
- Peptide Design
- Immunogen Design
- Antibody Purification
- Assay Development R&D
- Production, Sales, Marketing & Global Distribution
- Bulk & Custom Packaging
Custom Antibody Development
If you are looking to develop your own cell line or have a target of interest, learn more about our custom antibody services.
Learn More